
About “Liou Yih-Cherng”
- Bio
- Active grants
- My notes
Academic Qualifications
D. Phil. (Queen’s, Canada), B. Sc. (NTOU, Taiwan)
Major Research Interests
The phosphorylation of specific proteins on Ser/Thr residues preceding proline is thought to be a major cellular signaling mechanism; however, very little is known about how the phosphorylation actually regulates protein function. Pin1 is an 18 kDa protein with two domains: the N-terminal WW domain (named after two invariant Trp residues, amino acids 1-39) and the C-terminal PPIase domain (amino acids 45-163). The WW domain acts as a binding module to bind its substrate via the phosphoserine (p-Ser) or phosphothreonine (p-Thr)-proline motifs in its substrates. The C-terminal PPIase domain is the catalytic domain to isomerize the conformational changes of specific pSer/Thr-Pro motifs. Unlike other peptidyl-prolyl isomerases (PPIases), Pin1 is a unique peptidyl isomerase that recognizes only the phosphorylated Ser/Thr motif preceding a proline residue. In addition, Pin1 is very prominent in isomerizing the cis-trans conformation of prolyl-peptidyl bonds in its substrates, resulting in regulation of their biological functions, suggesting the conformational changes mediated by Pin1 or Pin1-like isomerase may be crucial for the normal functioning of cells.
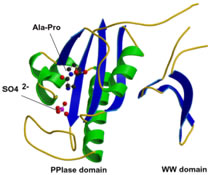
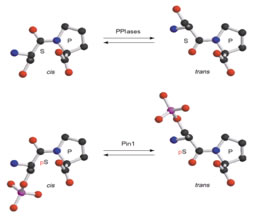
Our research directions lie in the area of how protein structure impacts signaling in the cells. The objectives are to characterize further the biology function of Pin1 and its downstream substrates. We are interested in understanding how the prolyl isomerase regulates its targets by changing the conformation on the pSer/Thr-Pro motifs in different organisms and in human diseases.

